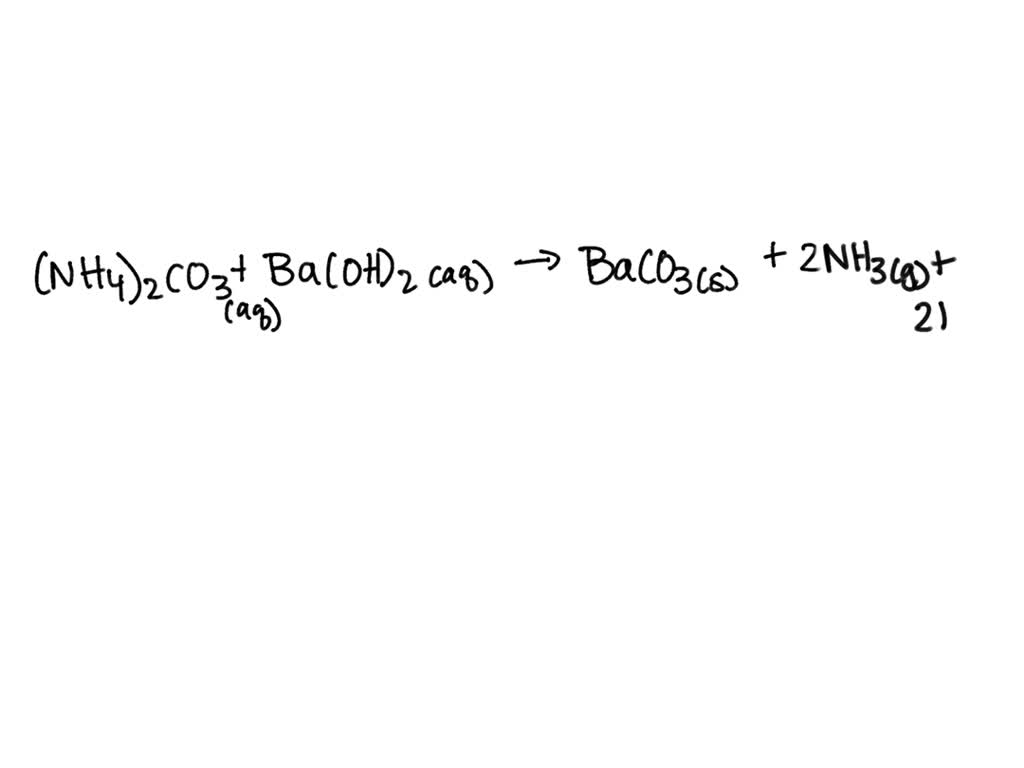
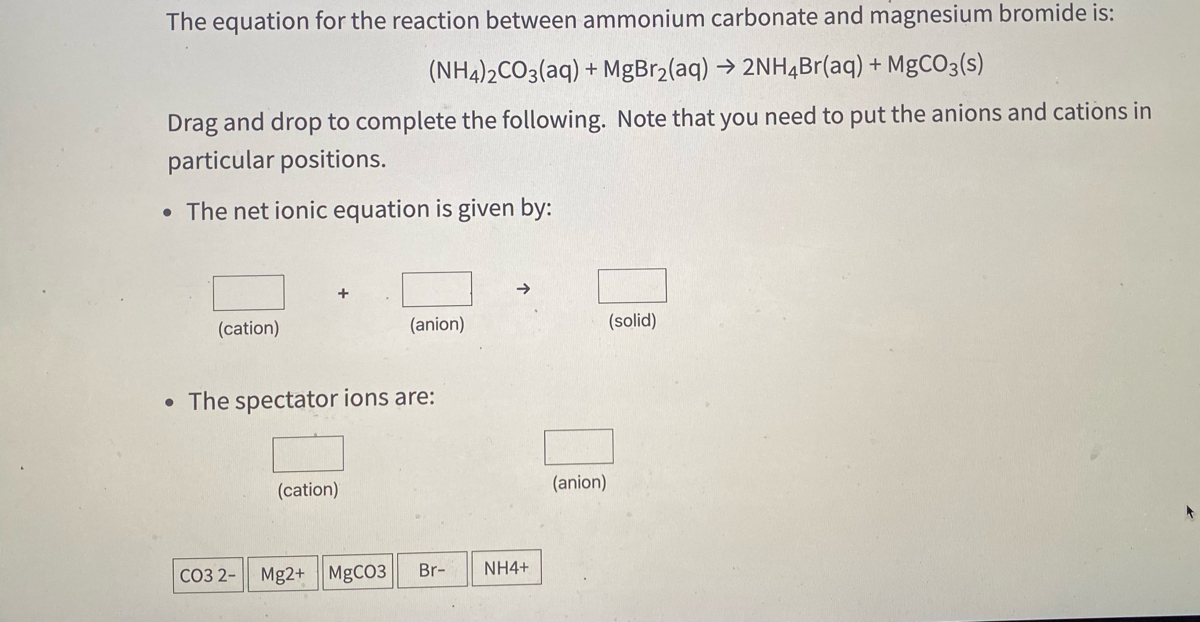
SOLVED: Which one of the following reaction equations is the net ionic equation for the reaction of ammonium carbonate solution with barium hydroxide solution? Group of answer choices 2NH4+(aq) + CO32-(aq) +

organic chemistry - Product of the reaction between hexane‐2,5‐dione and ammonium carbonate - Chemistry Stack Exchange

What is the formula of the compound formed between the ammonium ion and the carbonate ion? A. NH4CO3 B. NH4(CO3)2 C. (NH4)2CO3 D. (NH4)3CO3 | Homework.Study.com

SOLVED: When ammonium carbonate is heated, three gases are produced by its decomposition: (NH4)2CO3 â†' 2 NH3 (g) + CO2 (g) + H2O (g). If 9.0 g of ammonium carbonate is decomposed

CH3 - O ||C - CH2 - CH2 - O ||C - CH3 (NH4)2CO3 Δ→(A) CCl3CO2Na Δ→ (major)(B) Product (B) of given reaction is:

Ammonium Carbonate (506-87-6) ((NH4)2@CO3) - China Ammonium Carbonate, Baker's Ammonia | Made-in-China.com

Amazon.com: Spectrum A1154-500GM Ammonium Carbonate, Powder, Technical Grade, (NH4)2CO3, 10 cc : Industrial y Científico

![Ammonium carbonate - Optional[FTIR] - Spectrum - SpectraBase Ammonium carbonate - Optional[FTIR] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/I567hx3XQ8t/structure.png?h=300&w=382)


![Solved 1. [20 points] When heated ammonium carbonate | Chegg.com Solved 1. [20 points] When heated ammonium carbonate | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F8e6%2F8e6a7178-f042-4c3b-a7c7-cb9d1e98dddb%2FphpYG52J9.png)






![Ammonium Carbonate [(NH4)2CO3] Molecular Weight Calculation - Laboratory Notes Ammonium Carbonate [(NH4)2CO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/ammonium-carbonate-molecular-weight-calculation-300x198.jpg)

![ANSWERED] The chemical formula for ammonium carbonate is NH4 2... - Physics ANSWERED] The chemical formula for ammonium carbonate is NH4 2... - Physics](https://media.kunduz.com/media/sug-question-candidate/20220519055123753995-3486304.jpg)
