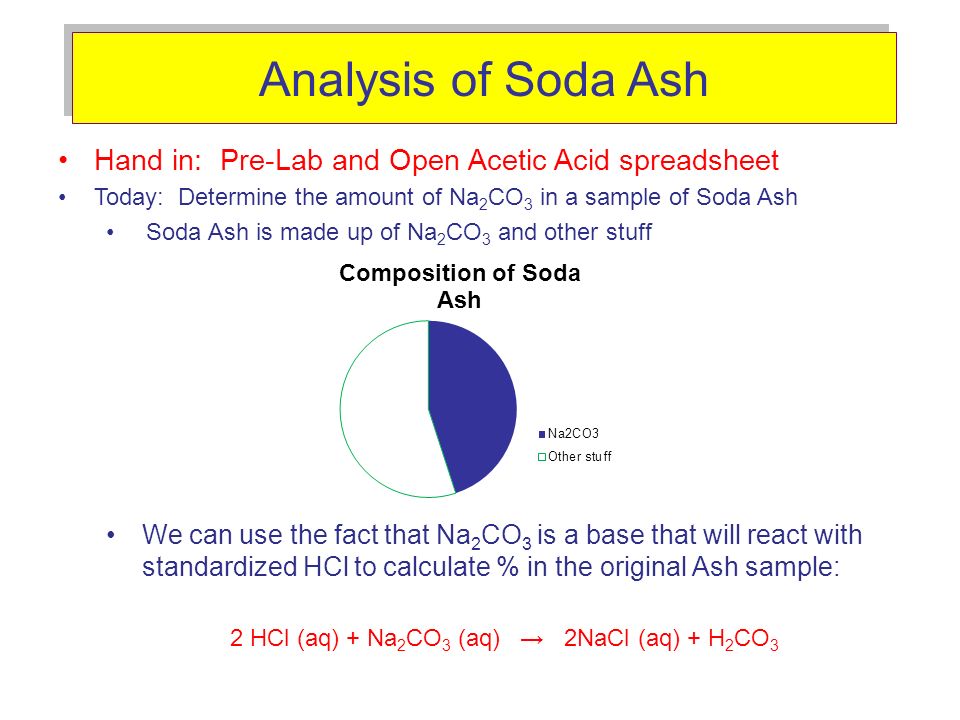
SOLVED: Consider the reaction between Na2CO3 and HCl: 2HCl(aq) + Na2CO3(aq) ⟶ H2O(l) + CO2(g) + 2NaCl(aq) When 16.3 g of HCl reacts with 17.2 g of Na2CO3, 4.33 g of CO2

SOLVED: Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This equation is balanced as written: 2HCl(aq)+Na2CO3(aq)→2NaCl(aq)+H2O(l)+CO2(g) Part A What volume of 1.75

Solved! How many liters of 0.53 M HCl is required to neutralize 0.78 g of sodium carbonate (Na2CO3)? (MM of Na2CO3 = 105. 99 g/mol) 𝟐𝑯𝑪𝒍 + 𝑵𝒂𝟐𝑪𝑶𝟑 → 𝟐𝑵𝒂𝑪𝒍 +

Na2CO3+HCl=NaCl+CO2+H2O Balanced Equation||Sodium carbonate+Hydrochloric acid=Sodium chloride+Carbon - YouTube
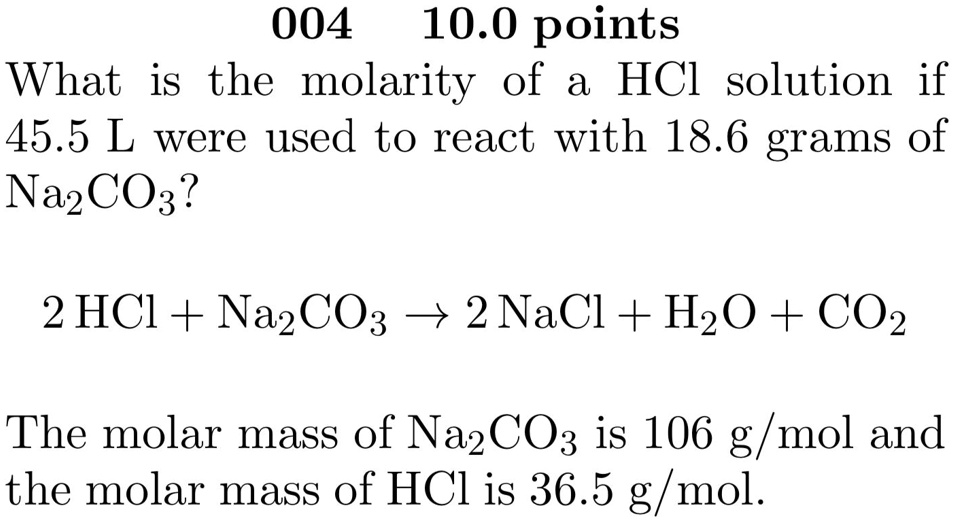
SOLVED: 004 10.0 points What is the molarity of a HCl solution if 45.5 L were used to react with 18.6 grams of Na24 CO3? 2 HCl + Na2CO3 2 NaCl +

Question Video: Determining the Products Formed from the Reaction between Sodium Carbonate and Hydrochloric Acid | Nagwa
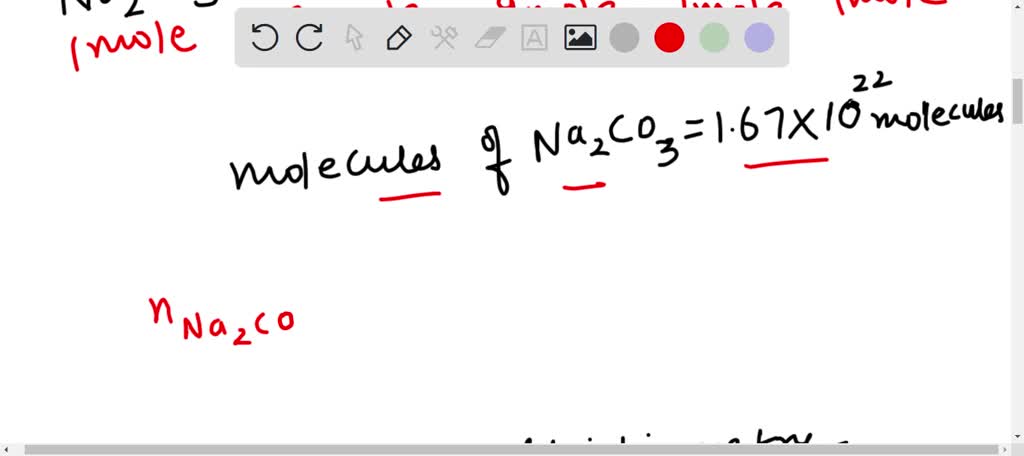
SOLVED: Na2CO3 + 2HCl ———> 2NaCl + CO2 + H2O How many moles of NaCl are produced from the reaction of 1.67 x 1022 molecules of Na2CO3 with excess HCl?

Welcome to Chem Zipper.com......: When 15 gm of NaCl and Na2CO3 is heated with dilute HCl, 2.5 gm of CO2 is evolved at NTP. Calculate percentage composition of the original mixture.

SOLVED: Consider the reaction: Na2CO3 + 2HCl â†' 2NaCl + CO2 + H2O. A minimum of moles of sodium carbonate is needed to fully react with 750 g of HCl.

The balanced equation is Na2CO3(s)+2HCl(aq)—>2NaCl(aq)+H2O(1)+CO2(g) The powder substance is 0.575The - Brainly.com

SOLVED: A reaction mixture of 18.215 g Na2CO3 and 10.938 g HCl react according to the following balanced equation: Na2CO3 + 2HCl â†' 2NaCl + H2O + CO2 If 12.75 g of

SOLVED: A reaction mixture of 18.215 g Na2CO3 and 10.938 g HCl react according to the following balanced equation: Na2CO3 + 2HCl → 2NaCl + H2O + CO2 What is the theoretical
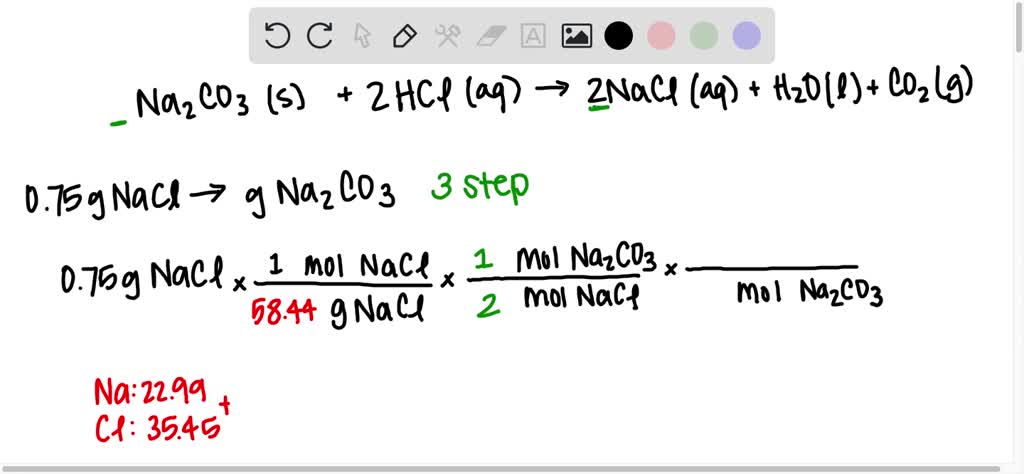
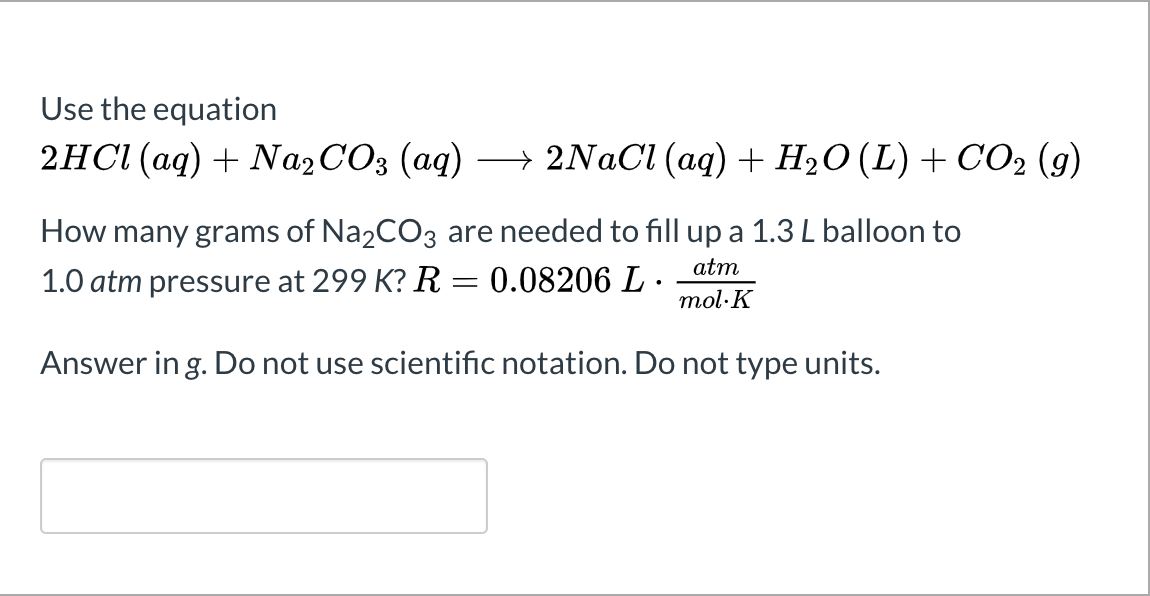
SOLVED: Na2CO3(s) + 2HCl(aq) â†' 2NaCl(aq) + H2O(l) + CO2(g) Using the balanced chemical reaction from step 1, calculate the mass of sodium carbonate (Na2CO3) that would be required to make 0.75

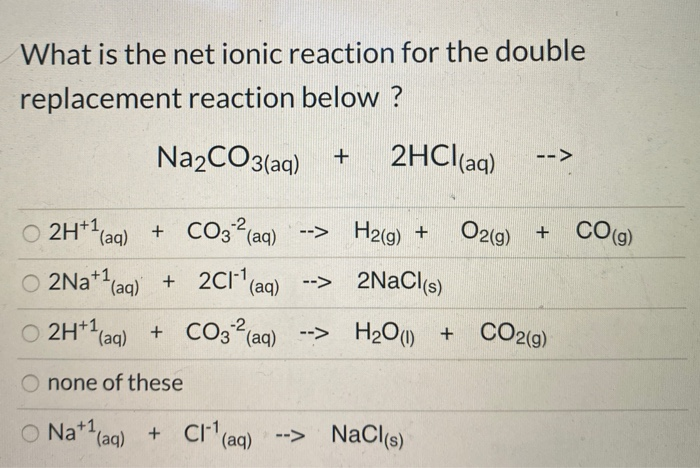
SOLVED: 2. Na2CO3(aq) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l) Balanced Equation: Complete Ionic Equation: Net Ionic Equation:


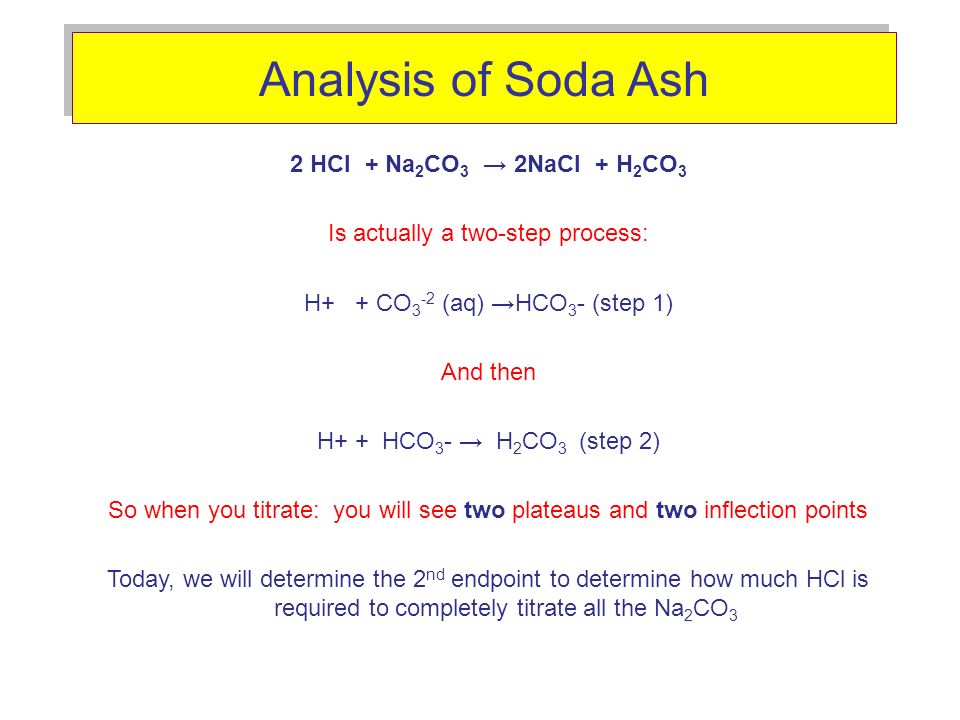
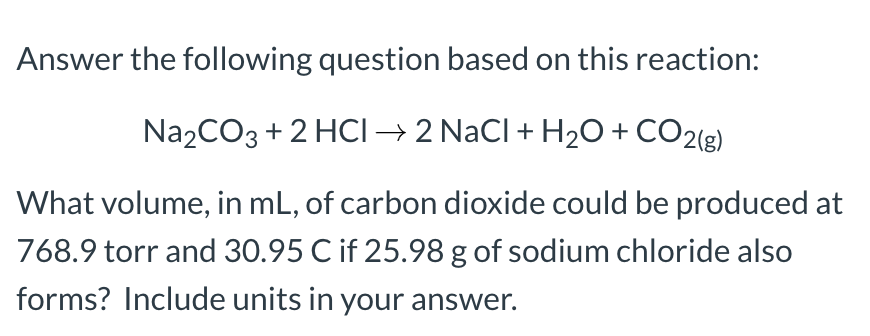


![Solved 0.32 M for 20 ml solution = [ Na2CO3] .9 Volume of | Chegg.com Solved 0.32 M for 20 ml solution = [ Na2CO3] .9 Volume of | Chegg.com](https://media.cheggcdn.com/study/ce6/ce696eaa-9751-4a1a-b5aa-f03d15d32ae3/image)