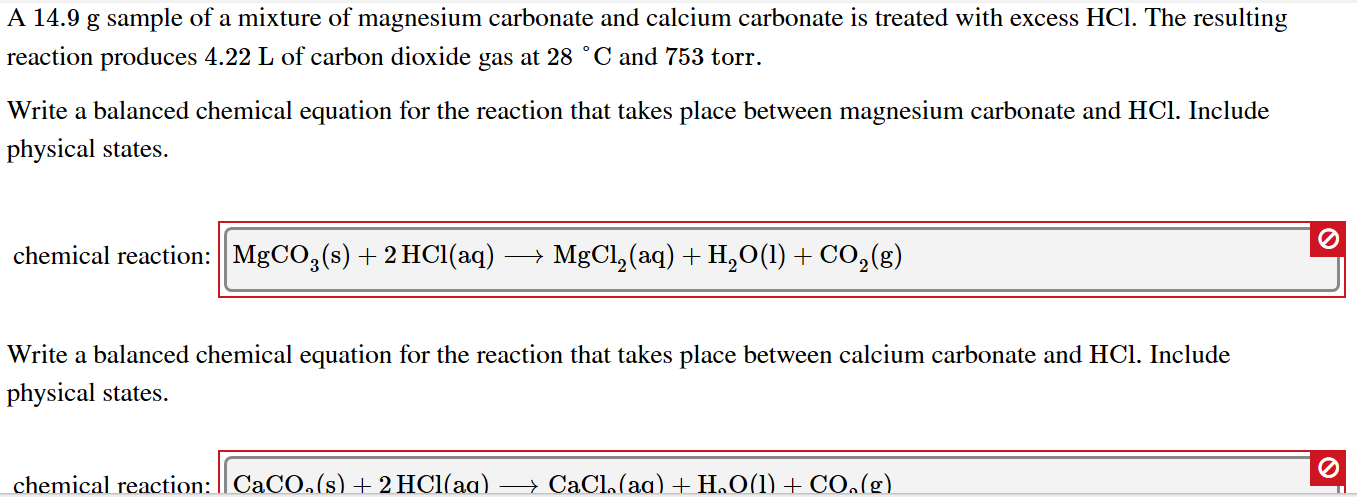
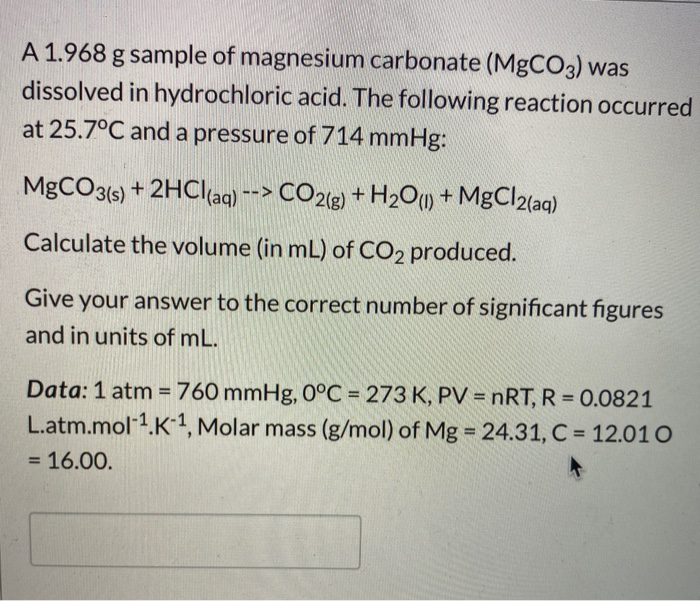
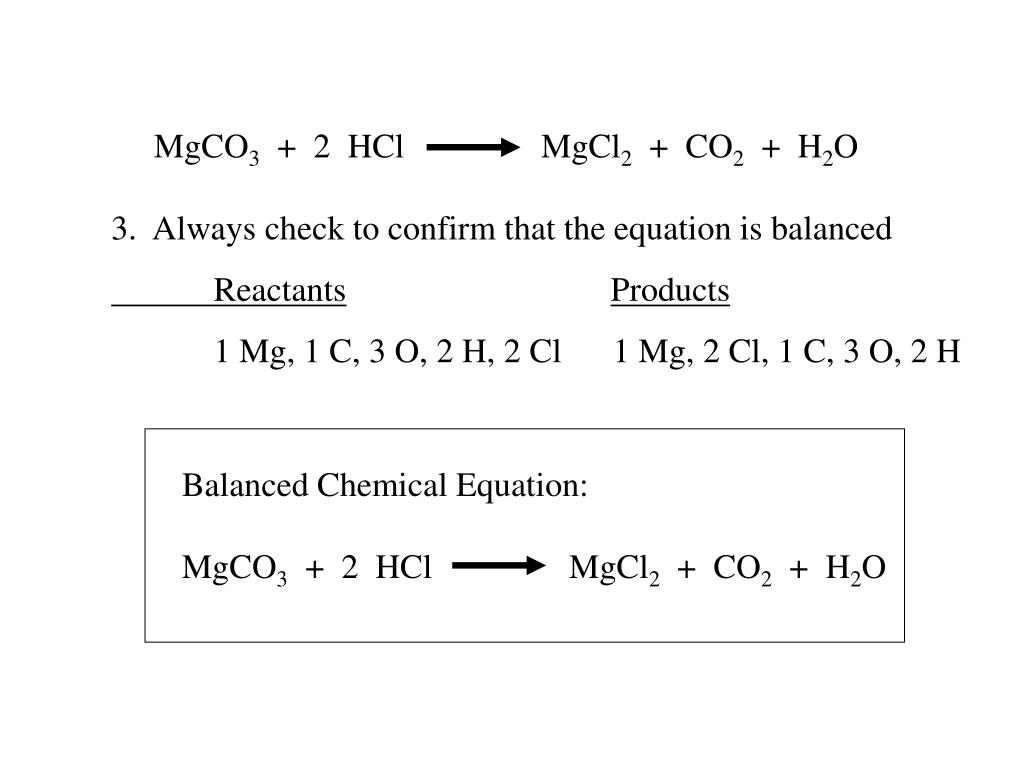
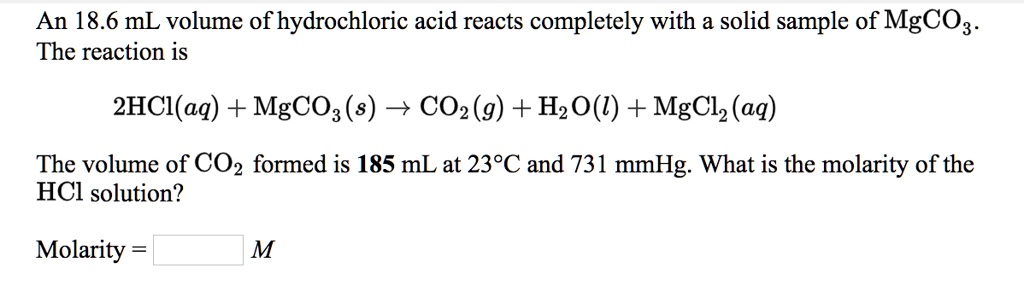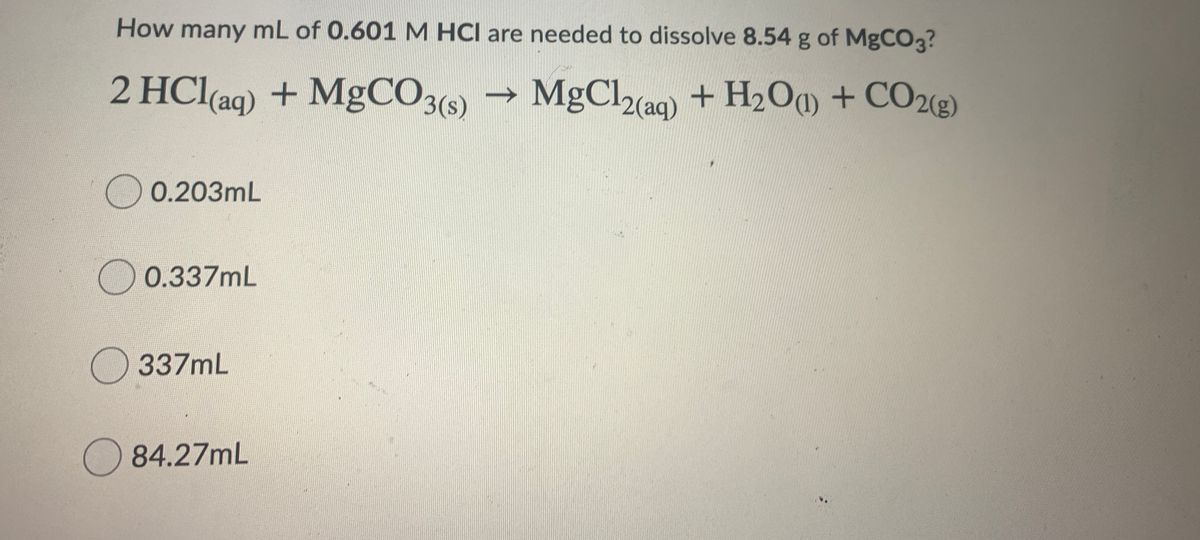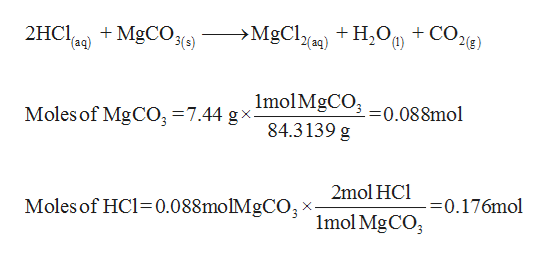How are we supposed to know that there's 2 compounds of HCl reacting? In other words, why is the product H2CO3 instead of HCO3- (because I'm reacting 1 HCl with 1 MgCO3) :

How to balance HCl+MgCO3=MgCl2+CO2+H2O|Chemical equation HCl+MgCO3=MgCl2+CO2+H2O| HCl+MgCO3= - YouTube
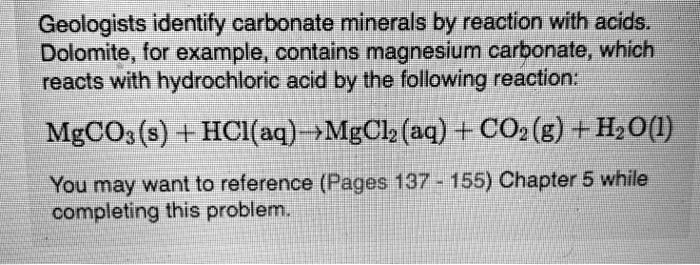
SOLVED: Geologists identify carbonate minerals by reaction with acids. Dolomite, for example, contains magnesium carbonate, which reacts with hydrochloric acid by the following reaction: MgCO3 (s) + HCl (aq) â†' MgCl2 (aq) +

If 20 g of CaCO3 is treated with 20 g of HCl, how many grams of CO2 can be generated according to the following equation ? CaCO3 + 2HCl → CaCl2 +

SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs

How are we supposed to know that there's 2 compounds of HCl reacting? In other words, why is the product H2CO3 instead of HCO3- (because I'm reacting 1 HCl with 1 MgCO3) :
1gm. Sample of Mgco3 is completely neutralise by n10 HCL (150ml.) .calculate the percentage purity of sample