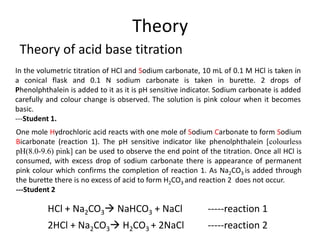
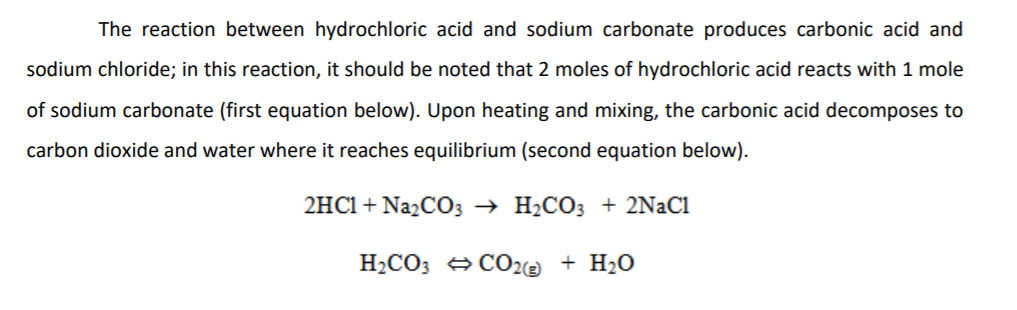
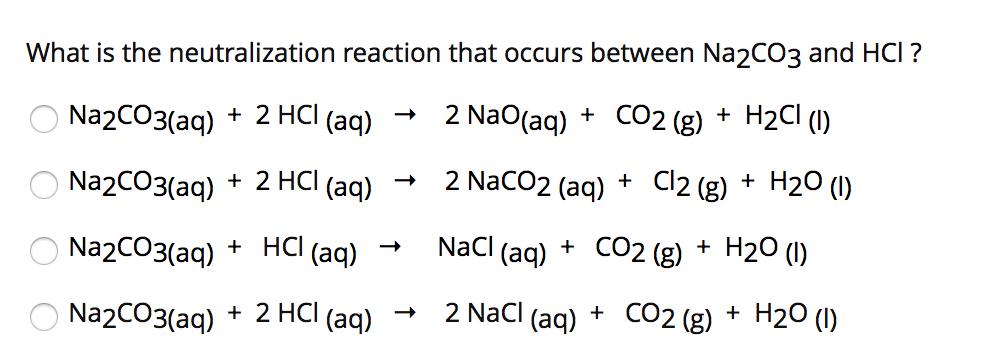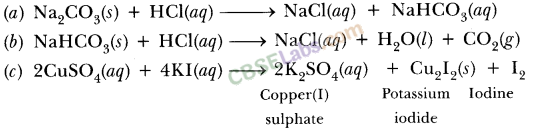Reaction of Hydrogen Chloride Gas with Sodium Carbonate and Its Deep Removal in a Fixed-Bed Reactor | Industrial & Engineering Chemistry Research

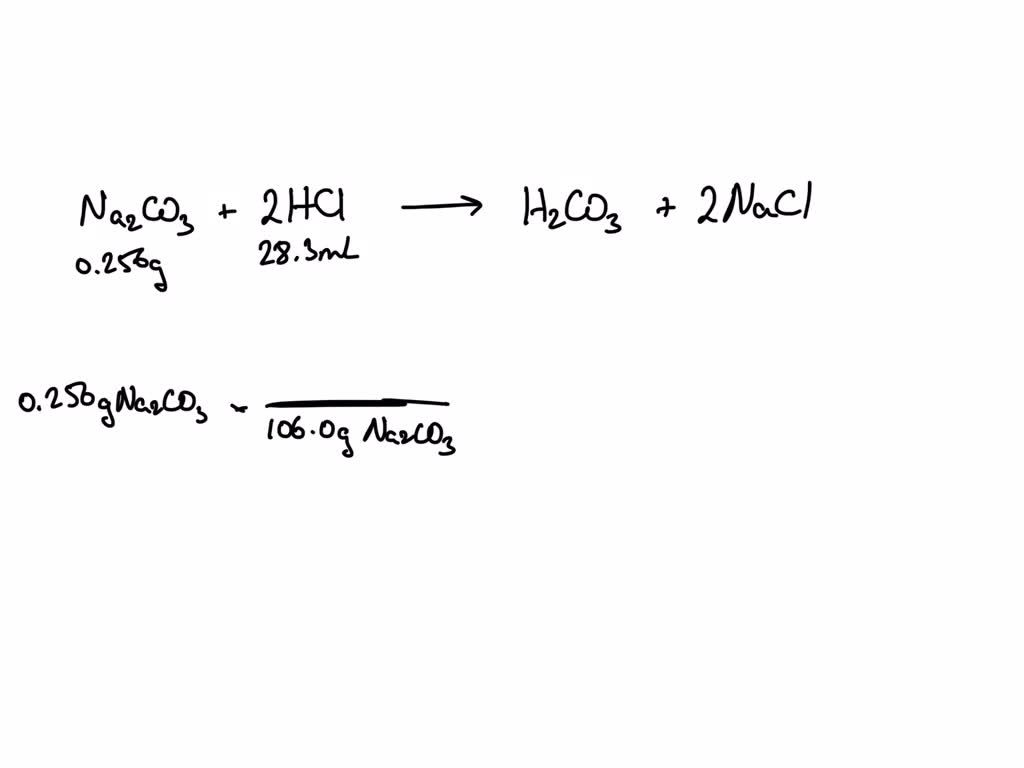
Solved! How many liters of 0.53 M HCl is required to neutralize 0.78 g of sodium carbonate (Na2CO3)? (MM of Na2CO3 = 105. 99 g/mol) 𝟐𝑯𝑪𝒍 + 𝑵𝒂𝟐𝑪𝑶𝟑 → 𝟐𝑵𝒂𝑪𝒍 +

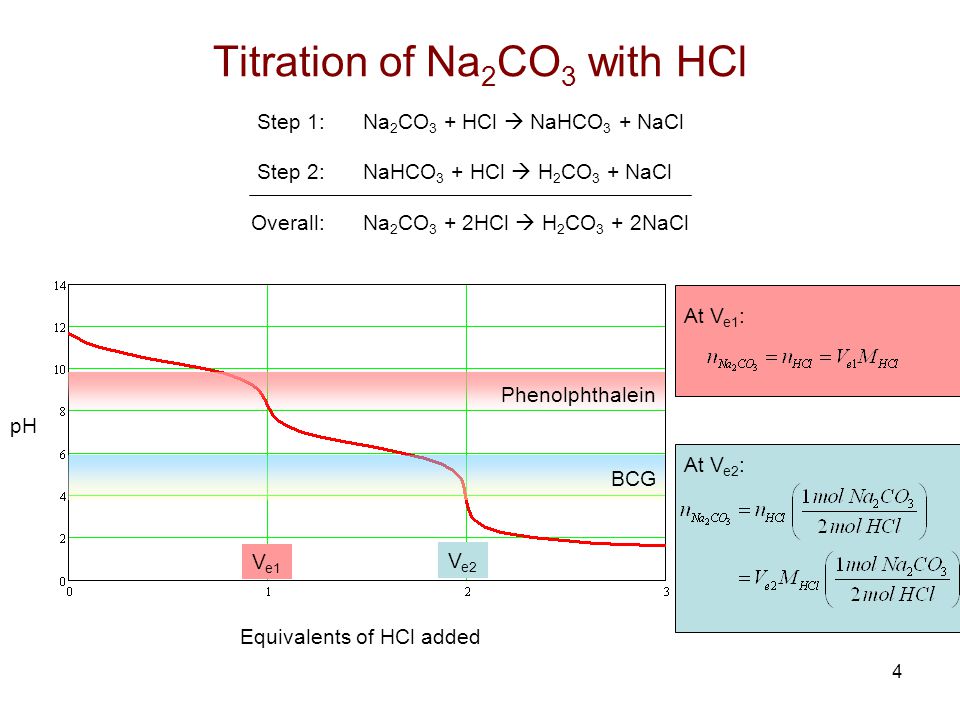
In the mixture of (NaHCO3 + Na2CO3) volume of HCl required is x mL with phenolphthalein indicator and y mL with methyl orange indicator in the same titration. Hence, volume for complete

Sodium Carbonate + Hydrochloric Acid - Na2CO3 + HCl - Molecular Equations & Net Ionic Equations - YouTube

The student will: be able to explain the experimental technique of titration. math calculate the molarity or volume of an unknown solution using the titration. - ppt download
What are reasons for getting two different concentration values of Na2CO3 when it was titrated with HCL using Phenolphthalein and Methyl Orange? - Quora

SOLVED: 2. Na2CO3(aq) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l) Balanced Equation: Complete Ionic Equation: Net Ionic Equation:

Question Video: Identifying the Observations of the Reaction between Hydrochloric Acid and Sodium Carbonate | Nagwa
What volume of 0.25M Hcl is required to react completely with 22.6g of Na2co3 according to the equation Na2Co3+2Hcl=2Nacl=H20 (2) The molecular mass of organic compound is 78